How Many Protons Neutrons and Electrons Are in Carbon 14
How is the charge on an anion determined. Its atomic mass is 14 amu six protons and eight neutrons.
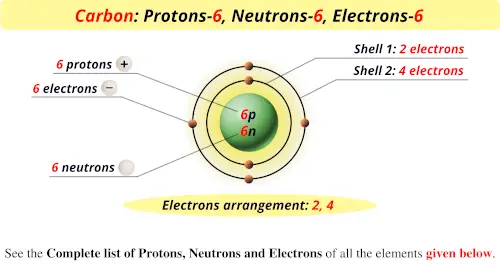
Protons Neutrons Electrons Of All Elements List Images
These two alternate forms of carbon are isotopes.
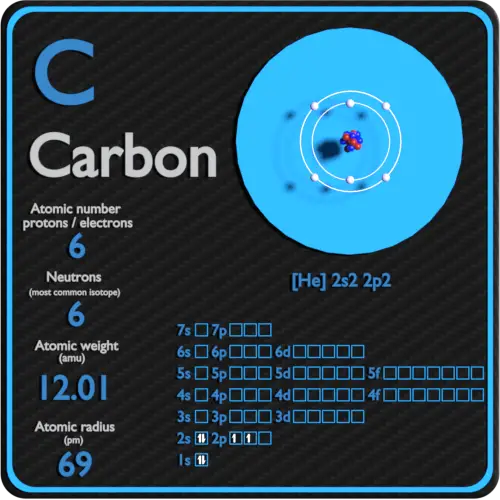
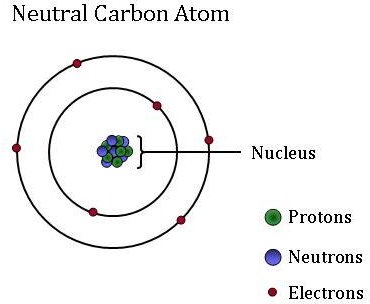
. Carbon-14 has 6 electrons. We call it carbon-14 because the total number of protons and neutrons in the nucleus also known as the mass number adds up to 14 6814. 6 electrons 8 neutrons 6 protons.
The 14C nuclide has 6 nuclear protons and 8 neutrons. How many protons neutrons and electrons are found in carbon-12. Primary Sidebar Recent Posts.
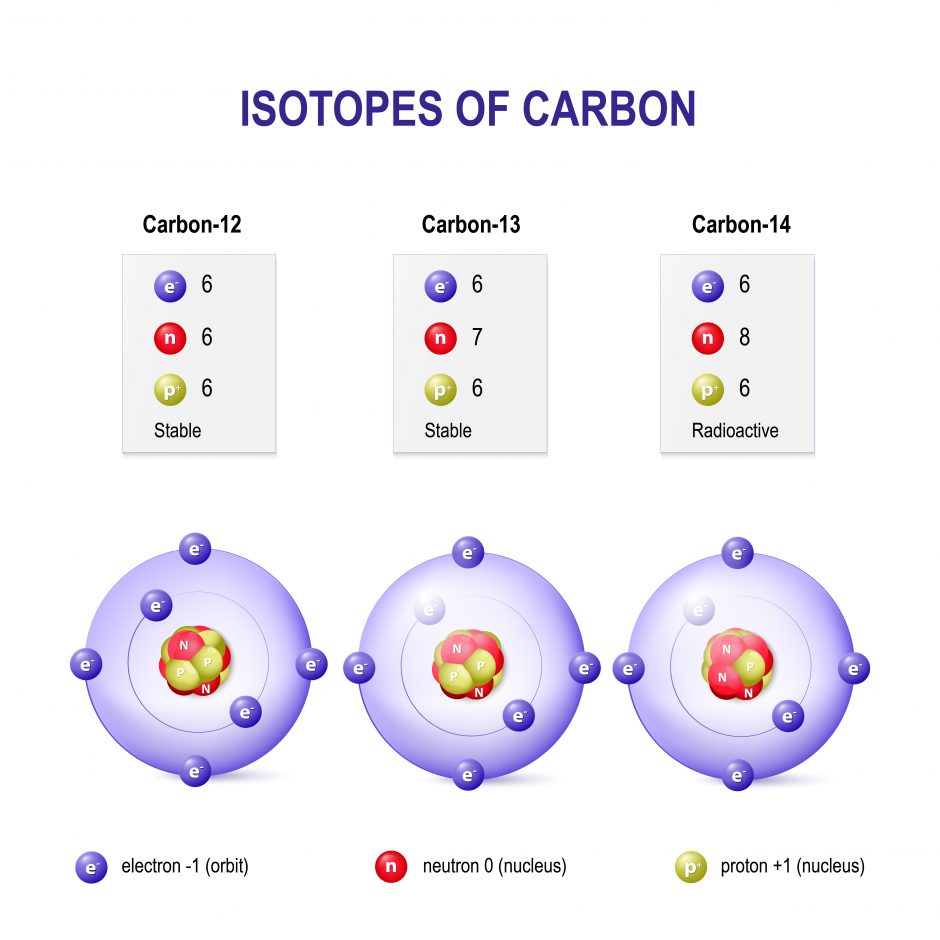
Correct option is D C 14 has atomic no 6 and mass no 14. Carbon 12 13 and 14 are carbon isotopes meaning that they have additional neutrons. Neutral carbon-14 contains six protons eight neutrons and six electrons.
12 C 13 C and 14 C are isotopes of carbon Z 6 and for that function have 6protons They in addition should have 6 electrons if the atoms are objectiveThe one difference in between these isotopes is the variety. ALL carbon nuclei contain 6 protons 6 positively charged massive nuclear particles. Isotopes are atoms with the same atomic number Z and a different mass number A.
In the carbon-14 isotope there are 6 protons 8 neutrons and 6 electrons. These are two examples of isotopes of carbon. We call it carbon-14 because the total number of protons and neutrons in the nucleus also known as the mass number adds up to 14 6814.
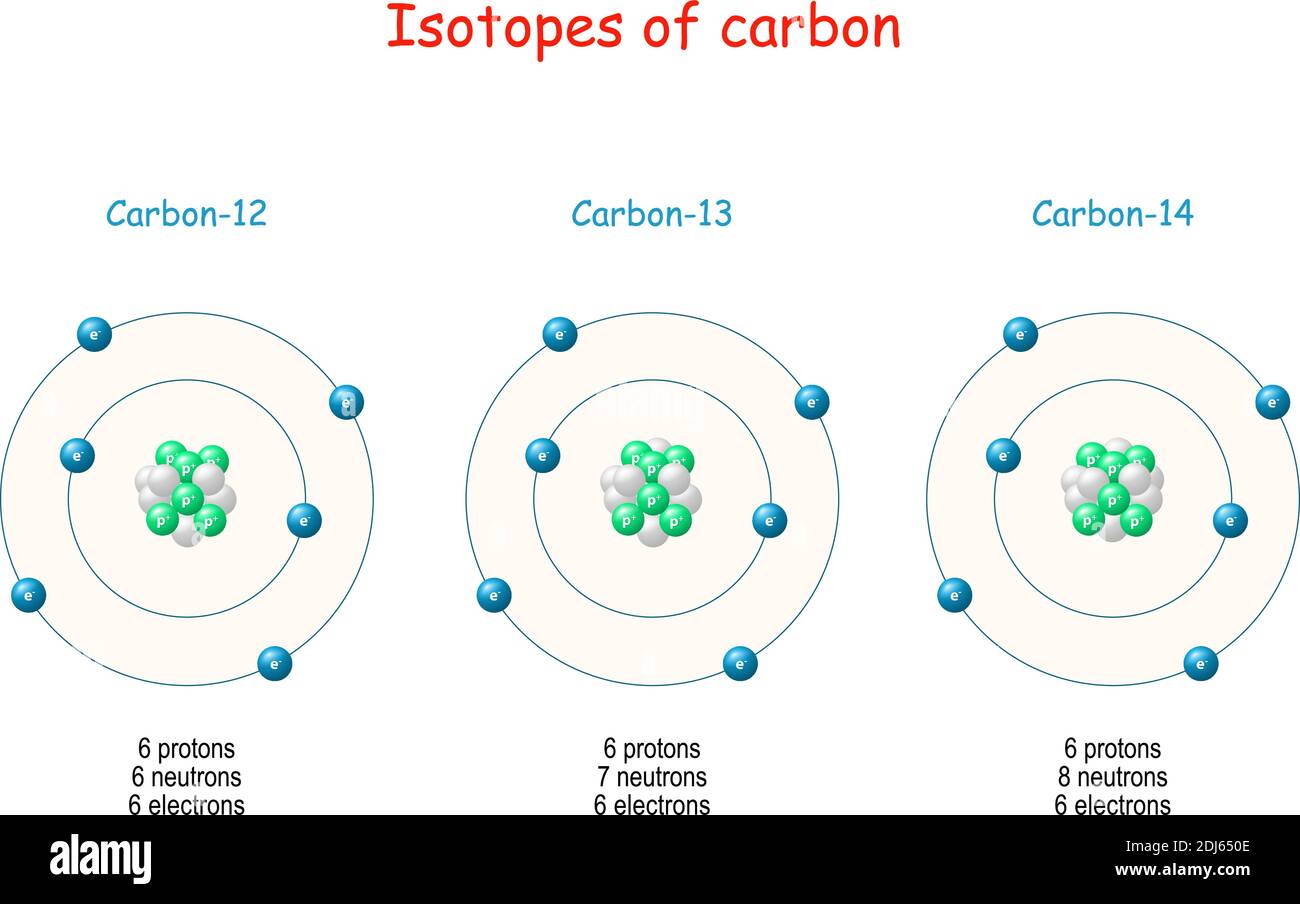
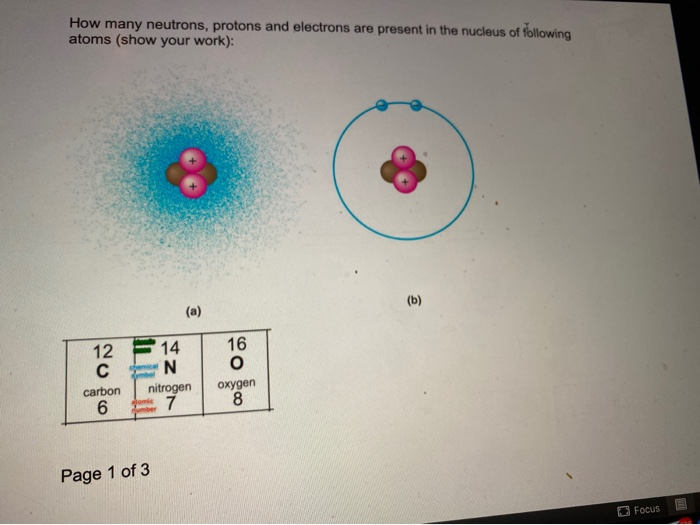
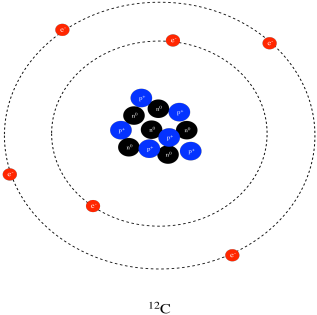
Carbon 12 has exactly 6 protons and 6 neutrons hence the 12 Carbon 13 has 6 protons and 7 neutrons. A carbon-14 atom has 6 protons 8 neutrons and 6 electrons so its mass number is 14 and its atomic number is 6. The number of neutrons varies with the isotope.
How many protons are in 10g of carbon-14. Its mass number is 14 six protons plus eight neutrons. What element has 6 protons 8 neutrons and 6 electrons.
Carbon is nonmetallic and tetravalentmaking four electrons available to form covalent chemical bonds. Carbon Cstart message C end message en masse 14 component has 4 electrons in its outershell Carbon generally shares electrons to attain a full valence shell generating bonds with many various various other atoms. Atoms of carbon-12 have 6 neutrons while atoms of carbon-14 contain 8 neutrons.
An atom of nitrogen by definition has exactly 7 protons. No of electrons No of protons atomic no 6. The various isotopes of an element are made a decision by developing the mass range of the atom within the prime left edge of the signal for the element.
For example carbon-14 is a radioactive isotope of carbon that has six protons and eight neutrons in its nucleus. The major economic use of carbon other than food and wood is in the form of hydrocarbons most notably the fossil fuel methane gas and crude oil. Draw the isotopes for each of the above.
3 rows Neutral carbon-14 contains six protons eight neutrons and six electrons. These two alternate forms of carbon are isotopes. Carbon Protons Neutrons Electrons Electron Configuration.
What are the differences between the mass number A the atomic number and the atomic mass of an element. Z the atomic number. For carbon-14 and carbon-12 how many protons and neutrons are in each nucleus.
Assuming neutral atoms how many electrons are present in an atom of carbon-14 and in an atom of carbon-12. Carbon is one of the few elements known since antiquity. That is they differ in the number of neutrons eg carbon 12 has 6 protons 6 electrons and 6 neutronsand carbon 14 8 neutrons 6 electrons and 6 protons.
For all atoms with no charge the number of electrons is equal to the number of protons. 7 electrons 7 protons and 7 neutrons. C 14 has atomic no 6 and mass no 14.
Carbon-14 or 14C contains six protons eight neutrons and six electrons. Its mass number. Is carbon 12 a proton.
For example carbon-14 is a radioactive isotope of carbon that has six protons and eight neutrons in its nucleus. A neutral atom would have the same number of protons and electrons so a neutral atom of carbon-12 or carbon-14 would have 6 electrons. How do we measure carbon footprint.
A neutral atom would have the same number of protons and electrons so a How Many Electrons Are In Carbon 13six electronsHow many electrons are in this amount of 13 Csix electronsC 13C and 14C are isotopes of carbon Z 6 and therefore contain six protons. No of electrons No of protons atomic no 6. No of neutron mass no-atomic no 146 8.
A neutral nitrogen atom will also have 7 electrons. Howmany electrons fill the outer shell of carbon. How are the number of neutrons.
The most common form of nitrogen has 7 neutrons which makes up 99 of the nitrogen atoms on earth although nitrogen atoms with 8 neutrons can also be found. The 14C nuclide has 6 nuclear protons and 8 neutrons How do you know. Well it is a carbon nucleus by specification.
How is the charge on a cation determined. No of neutron mass no-atomic no 146 8.
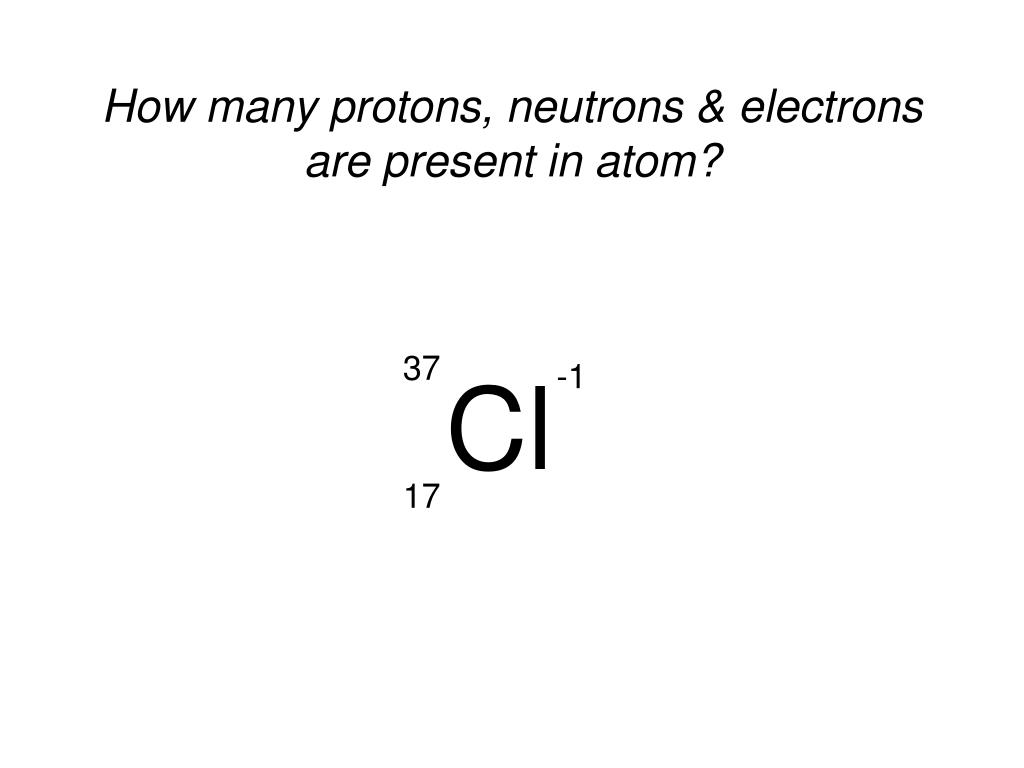
Ppt How Many Protons Neutrons Electrons Are Present In Atom Powerpoint Presentation Id 297413
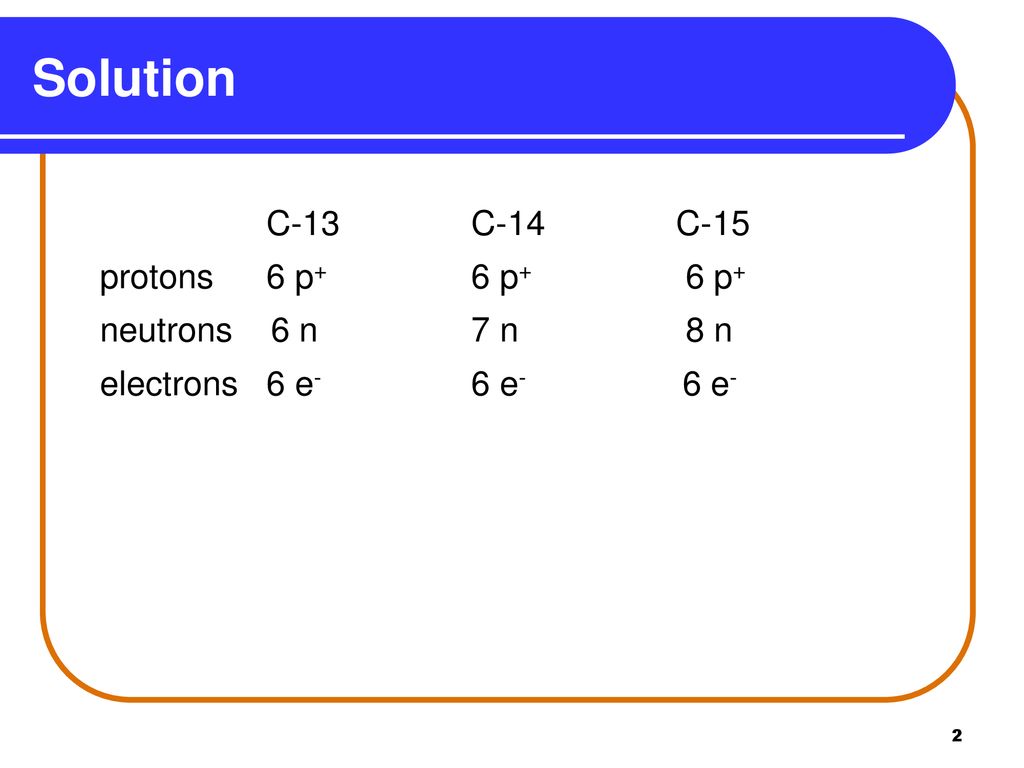
Learning Check Naturally Occurring Carbon Consists Of Three Isotopes Carbon 13 Carbon 14 And Carbon 15 State The Number Of Protons Neutrons And Ppt Download
How Many Electrons Does Carbon 14 Have Quora

Isotopes Images Stock Photos Vectors Shutterstock
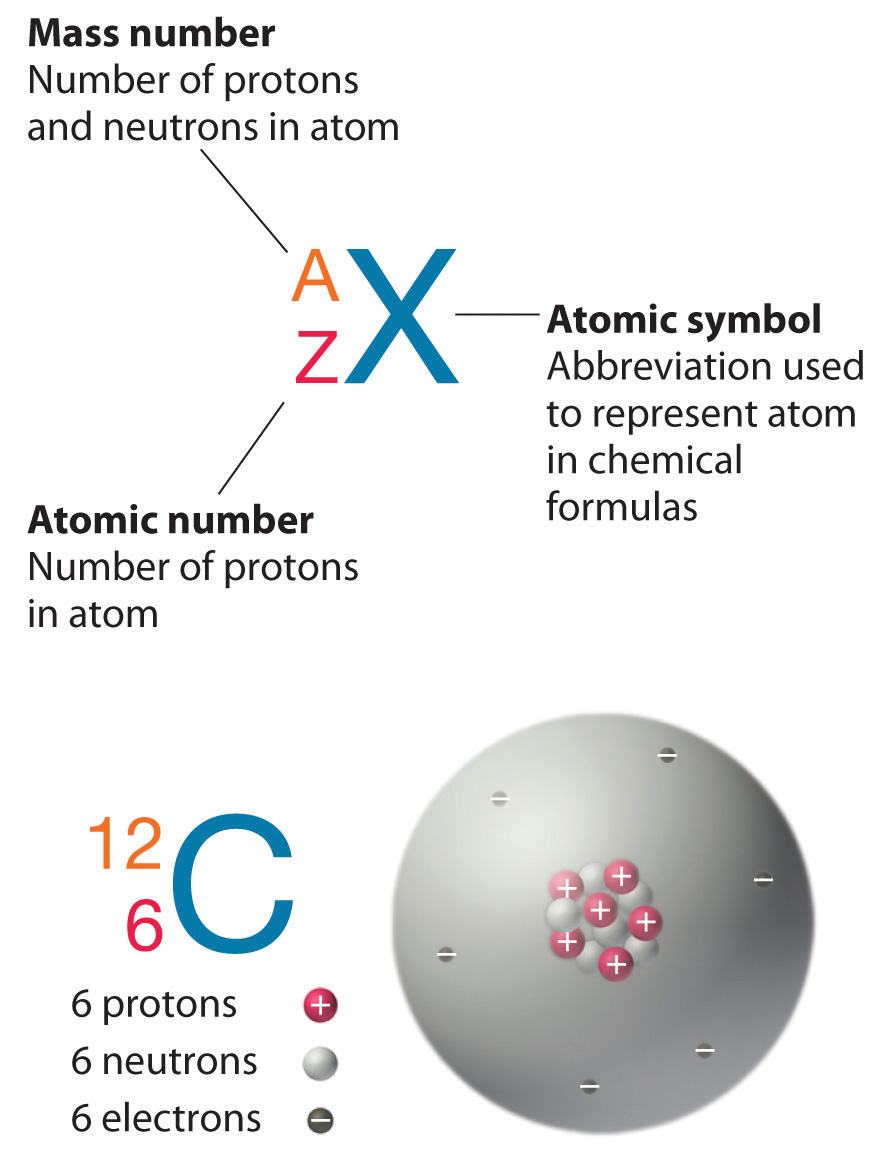
3 3 Isotopes Chemistry Libretexts

How Many Electrons Are In Carbon Quora

How To Find The Number Of Protons Electrons Neutrons For Carbon C Youtube
Atomic Structure Of Carbon High Resolution Stock Photography And Images Alamy
Concept Explanation Genchem Hub Under Construction

Carbon Protons Neutrons Electrons Electron Configuration

Vektor Stok Carbon Isotopes Atomic Structure Carbon12 Carbon14 Tanpa Royalti 1801162453

Carbon Isotopes Atomic Structure From Carbon 12 To Carbon 14 Atomic Particles Protons Neutrons Electrons Vector Illustration For Science Stock Vector Image Art Alamy
Drawing Atoms Montessori Muddle
Solved How Many Neutrons Protons And Electrons Are Present Chegg Com
Global Monitoring Laboratory Carbon Cycle Greenhouse Gases

How Many Protons Does Carbon Dioxide Have How Many Protons Neutrons And Electrons Does Carbon Have Lisbdnet Com
Structure Reactivity Atoms Protons Neutrons Electrons

Vektor Stok Carbon Isotopes Atomic Structure Carbon12 Carbon14 Tanpa Royalti 1801162453

Comments
Post a Comment